Gene Therapy
R&D of regenerative medicine employing engineered human adipocytes
CellGenTech, Inc.
President & CEO Masayuki Aso
[Address] Chiba-dai Inohana Innovation Plaza #101, 1-8-15, Inohana, Chuo-ku,Chiba-city, Chiba, Japan 260-0856
[URL] http://www.cellgentech.com/
[Classification] R&D of regenerative medicine employing engineered human adipocytes
[Business Summary]
We have been developing innovative cell/tissue-engineered products, namely, therapeutic gene-transduced adipocytes, for sustained release of the relevant enzyme or protein in vivo as a replacement therapy. The therapeutic gene is transferred to the patient’s adipocytes in vitro and the transduced cells are implanted back to the patient. Adipocytes have a long life span which may contribute to the long-lasting therapeutic effects over years.
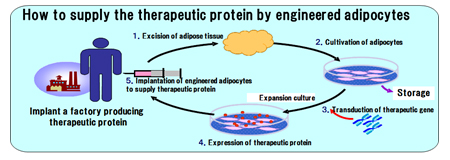
Our products are manufactured from the mature human adipocytes under the appropriate quality control with high safety concern. Our technical know-how on engineered adipocytes products can be expand to various intractable diseases. Our patent “Primarily cultured adipocytes for gene therapy” was granted in 39 countries over the world, and CGT has an exclusive license.
We are making effort to develop engineered adipocytes products as cell/gene therapy or regenerative medicine to combat against the disease and improve the QOL of the patients, as a pioneer of this field.
Currently, we are focusing on familiar LCAT deficiency for which no effective therapy is available until now. Through establishing the effectiveness of our engineered adipocytes products on familiar LCAT deficiency (Proof of Concept), we will step forward for early supply of our products to the bed side, that will be followed by further expansion of the R & D to lysosome disease and hemophilia, where the contribution of cell based medicine is expected. Furthermore, we will challenge to apply our technology in the field of neurodegenerative disease and cancer in future.
LCAT gene-transduced human adipocytes are employed in our on-going clinical research for familiar LCAT deficiency, and after the following clinical trial for clinical evaluation we are planning to file for marketing license in Japan as cell/tissue engineered products. Then clinical research/clinical trial for lysosome disease and hemophilia will be our next targets.









