Gene Therapy
Cancer Immune Therapy Research by Nagoya University Graduate School of Medicine
Cancer Immune Therapy Research Center
Our laboratory is interested in discovering new therapeutic methods against advanced cancer based on host anti-cancer immunity. Our interests focus in two main topics which include oncolytic virus and anti-cancer cell therapy.
We are using natural mutated herpes simplex virus, HF10 which has been studied in USA as well as in Japan. This oncolytic virus is well known for its effect on “continuous replication with infection of neighboring cells and killing cancer cells without any damage to normal cells”. HF10 is drastically different from former virus vectors. Recent studies have shown that oncolytic virus affects host immune response against specific cancer antigens, therefore it inhibits systemic metastasis.
As for cell therapy, we are interested in genetically modified receptor T-cell lymphocytes, which target cancer specific antigens on cancer cell surface. Our laboratory is studying a new generation technology for genetically modified receptor T-cell lymphocyte using iPS. We are amassing basic research data and eager to make a bridge to clinical trials with the goal of making a substantial difference in each patient’s prognosis.
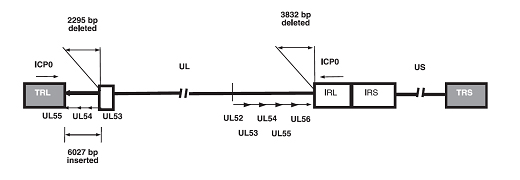
The development of oncolytic viruses has led to an emerging new class of cancer therapies. Most of the oncolytic herpes simplex viruses (HSVs) exhibit a high safety profile achieved through attenuation. HF10 is attenuated, replication-competent mutant strain of HSV-1 and displays strong tumor killing activity in vivo and in vitro. The effects of HF10 in clinic have been studied in various types of malignant tumors, including breast, pancreas, head & neck cancer, malignant melanoma. The aims of researches in our laboratory are to understand the complex interactions among the virus, the tumor, its microenvironment, and the host immune response. In particular, we will focus on mechanisms of action: direct cytotoxicity and/or immune activation by HF10 and on clinical development of HF10. We are focused on 3 main topics in our laboratory:
Because of the high degree of cancer clonal heterogeneity, intratumor genetic heterogeneity and cell signal complexity, current therapies are limited in exerting an ideal antitumor effect. In addition, studies with oncolytic HSV indicate that the oncolytic virus alone is not sufficient to destroy all tumor cells. To overcome the limitation, we evaluate antitumor effects of combination therapies to various cancers with HF10 and conventional chemotherapeutic agent or with HF10 and immune-checkpoint inhibitors, which have an entirely different anti-tumor mechanism from that of molecular targeted drugs or cytotoxic drugs. Treatment of tumors with immune-checkpoint inhibitors, monoclonal antibodies to programed cell death 1 (PD-1) or its ligand, (PD-L1) have resulted in significant clinical responses across multiple tumor types. The ‘in situ vaccination’ immunotherapy strategy directly manipulates identified tumors to overcome local tumor-mediated immunosuppression and subsequently stimulates systemic antitumor immunity by blocking of association between PD-1 and PD-L1. We have shown that intratumoral (IT) inoculation with HF10 resulted in a significant reduction in tumor growth and simultaneously generates potent systemic antitumor activity against non-inoculated tumor, suggesting that the treatment of tumor with HF10 induced a specific and systemic antitumor immune response. Strong antitumor effects of combination therapies to various cancers with HF10 and immune-checkpoint inhibitors are expected by inducing a strong and specific antitumoral immune response.
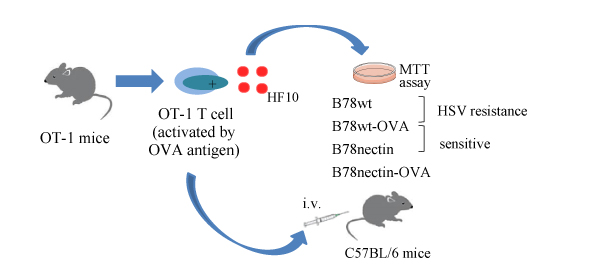
Most clinical trials using oncolytic viral therapy have been performed using direct IT injection. IT injection of oncolytic virus has shown disease response in injected and non-injected tumors and have entered late-phase clinical trials. However, IT administration is not possible in all cancer patients. Systemic administration (oral and IV) of virus has the obvious advantage of potentially reaching all sites of systemic disease, although the viruses are vulnerable to inactivation by antibody and related complement proteins, uptake by the reticuloendothelial system and neutralization by circulating antibodies. Therefore, creating viral delivery system with good efficiency and safety profile is a meaningful work which is required. Human peritoneal mesothelial cells were suitable for protecting viral particles within the circulation from neutralizing antibodies and ensuring tumor delivery. We focus on antigen-specific cytotoxic T cell as a delivery carrier of HF10. This study supports that T cells and HF10 work synergistically with the T cells improving the delivery of HF10; therefore producing an improved combined cytotoxic and oncolytic effect from both. Antigen-specific cytotoxic T cells could be promising HF10 delivery cells for cancer targeted therapy.
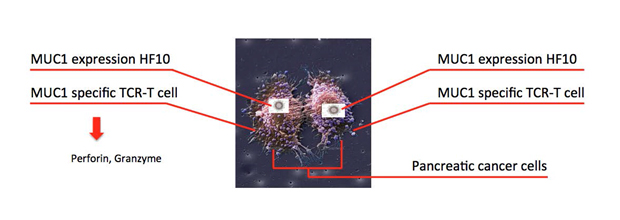
The adoptive transfer of tumor-reactive cells is a promising approach for the treatment of various cancers. Engineered TCRs T cells and chimeric antigen-receptors (CARs) T cells are an effective immune therapy for tumors. To enhance antitumor effect of engineered TCR T cells or CAR T cells, HF10 is used for a vector to express an antigen that recognized by engineered TCR T cells or CAR T cells. Direct viral infection of solid tumors can induce cell death of tumor, but it offers the delivery system to express exogenous factors to enhance the antitumor response. Exogenous expression of GM-CSF by an HSV amplicon could potentially promote the induction of an antitumor immune response to enhance the oncolytic effects of HSV. Genetically modified viruses have become important potential vectors for cancer therapy. Thus, we generated various antigens expressing HF10 by genetic modification method. Combination of genetically modified HF10 with engineered TCR T cells or CAR T cells most likely will become an important therapy for cancer.
Although engineered TCR T cells and CAR T cells therapy are powerful approach to cancer treatment, a very limited effective list of targets and safe T-cell adoptive immunotherapy limits treatment of solid cancer. We are keen to identify new target for effective immunotherapy and provide an improved and safer clinical treatment for patients with various malignancies.